Relevance: GS Paper III
News Excerpt:
The Central Drugs Standard Control Organisation (CDSCO) has granted market authorization to India's breakthrough Chimeric Antigen Receptor (CAR) T-cell therapy for patients with B-cell lymphomas who didn't respond to standard treatments like chemotherapy.

About CAR-T Cell Therapy
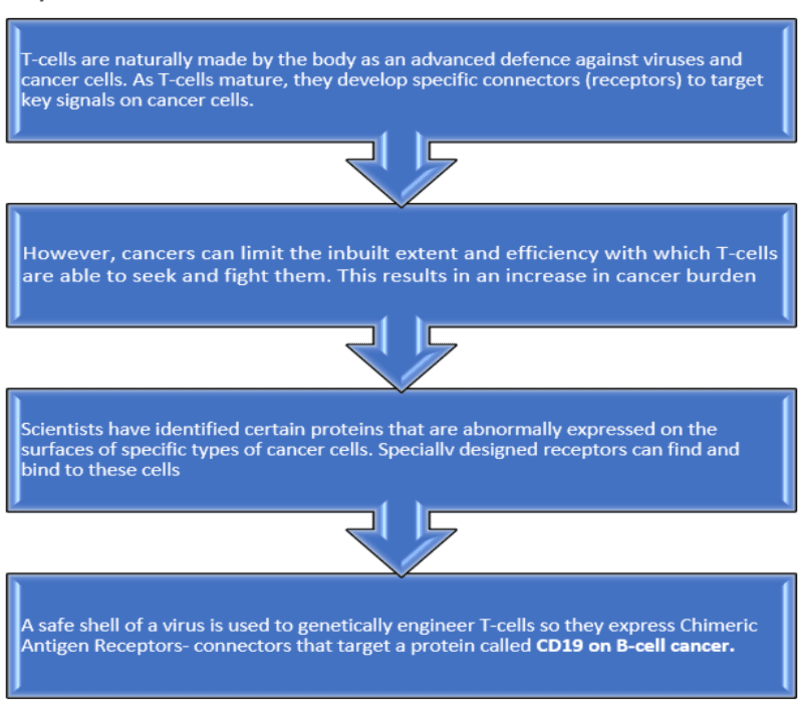
- CAR-T is a revolutionary therapy that modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- (CAR)- T cell therapy involves genetic modification of a patient's autologous T-cells in a laboratory so that they will bind to specific proteins (Antigens) on cancer cells and kill them.
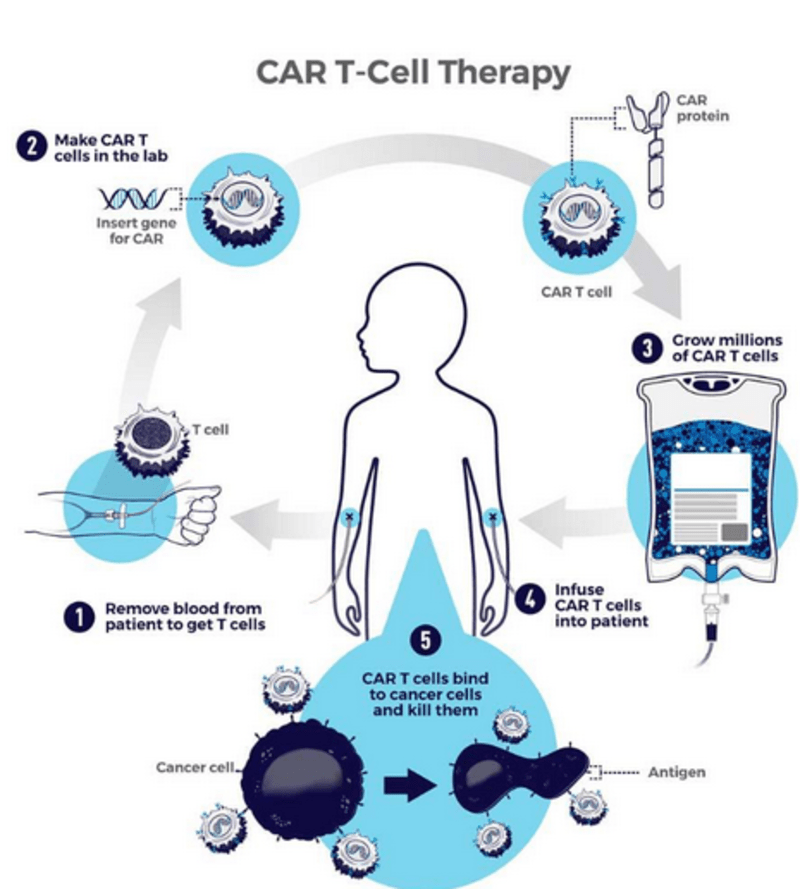
- The Process involves:
- A patient's T cells are removed from their blood.
- The gene for a particular receptor called a chimeric antigen receptor (CAR) is inserted into the T cells in the laboratory. The gene encodes the engineered CAR protein that is expressed on the surface of the patient's T cells, creating a CAR-T cell.
- Millions of CAR-T cells are grown in the laboratory.
- They are then given to the patient by intravenous infusion.
- The CAR-T cells bind to antigens on the cancer cells and Kill them.
NexCar19: a type of CAR-T indigenously in India-
- The Food and Drug Administration (FDA) has approved six CAR-T-cell treatments since 2017. All are licensed to treat blood cancers such as lymphomas, leukaemia, and, most recently, multiple myeloma.
- NexCar19 is a type of CAR-T and gene therapy developed indigenously in India by ImmunoACT and Tata Memorial Centre (TMC). ImmunoACT is a company incubated at IIT Bombay.
- Immunoadoptive Cell Therapy Private Limited (ImmunoACT) obtained CDSDO approval for NexCAR19, a CAR-T treatment, to treat relapsed or refractory B-cell lymphoblastic leukaemia.
- The approval came after a Phase I/II trial of 60 patients showed a 70% overall response rate and significant delay in cancer progression.

How NexCar19 Works?
- The Patient's white blood cells are extracted by a machine through leukapheresis and genetically modified, equipping them with the tools to identify and destroy the cancer cells.
- NexCar19 is manufactured to an optimal dose for a patient and is typically administered as a single intravenous infusion. Before this, the patient is put through chemotherapy to prime the body for the therapy.
The process:
Way Forward:
- Even some developed nations don’t have CAR-T therapies; they import them from the United States or Europe. India is now one of the first developing countries to have its indigenous CAR-T cell and gene therapy platform.
- CD19-targeted CAR-T cells also offer hope to adults and children with advanced aggressive lymphomas. This therapy can show tremendous results to those patients whose cancers return or relapse after chemotherapy or a stem-cell transplant.
- ImmunoACT is now poised to become the country's leading multi-product, multi-service cell and gene therapy platform that can significantly change the healthcare paradigm, especially in unmet needs.
