RAPID ANTIGEN DETECTION TEST
A rapid antigen test also called rapid diagnostic test used for point-of-care testing which directly detects the presence or absence of an antigen.
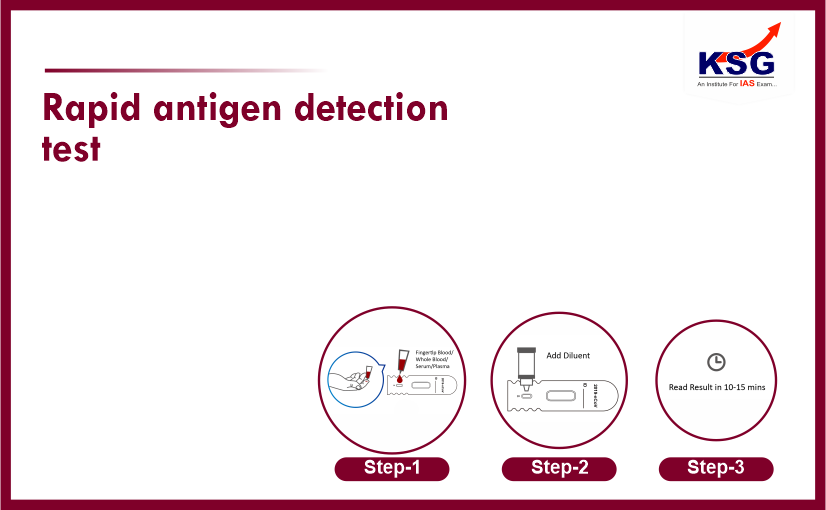
RAPID ANTIGEN DETECTION TEST
A rapid antigen test also called rapid diagnostic test used for point-of-care testing which directly detects the presence or absence of an antigen.
Rapid-antigen test is a test on swabbed nasal samples that detects antigens (foreign substance that induce an immune response in the body) that are found on or within SARS-CoV-2 virus.
WHY IS IN NEWS?
Recently, the Indian Council of Medical Research (ICMR) recommended the use of one more test for the diagnosis of COVID-19. The rapid antigen detection test is to be used in specified settings and containment zones or hotspots, and kits from only one manufacturer have approvals.
In India, the ICMR has allowed the South Korean company SD Biosensor which has a manufacturing unit in the Manesar to develop the antigen detection kit. The kit is commercially called Standard Q-COVID-19Ag detection kit, which is an inbuilt COVID antigen test device, used for viral extraction tube with viral lysis buffer and sterile swab for sample collection.
PATHAKCATCH name test kit is the first Indian made test kit that got approval recently, manufactured by Pune based Mylab Discovery Solutions.
The ICMR, has recommended that this test should be performed under strict supervision.
OBJECTIVE
COVID-19 tests detect either the coronavirus or antibodies made as part of the immune response to it. The Rapid Antigen Test is different from RT-PCR test. The RT-PCR was considered as the gold standard test for COVID19.
The major difference between both tests is TIME.
The RT-PCR test takes a minimum 2-5 hours including the time taken for the sample transportation. In a reliable antigen detection test, The maximum duration for interpreting a positive and a negative test is 30 minutes.
A rapid antigen test seeks to detect viruses similar to the RT-PCR test however the mechanism is different.
IMPORTANCE
The antigen test needs to be conducted at all the symptomatic influenza like illnesses in healthcare settings, hotspot and containment zones. In a healthcare environment it is appropriate in three groups.
First in all individual displaying influenza - like symptoms in a healthcare setting and suspected of having COVID 19 infection. Second, in the asymptomatic patients who are hospitalised or seeking hospitalisation for the following high risk conditions- such chemotherapy ,immuno-suppressed patients like those who are HIV positive ,patients diagnosed with malignant disease ,transplant patients elderly patients ( over age 65) with comorbidities —and Third, in asymptomatic patients undergoing aerosol-generating surgical / non-surgical treatment such as elective/emergency surgical procedures including neurosurgery, ENT surgery dental procedure, and non-surgical interventions like bronchoscopy and dialysis or those who have influenza-like symptoms suspected as having COVID-19 in a healthcare setting.
According to ICMR guidelines if the result of the test is positive it should be considered as true positive. However, those who are negative in this rapid antigen test should be tested by RT-PCR test, to prevent possible spread of the virus due to false negative.
ICMR plays an important role in this process, which is the apex body in India for the promotion coordination and formulation of biomedical research.
