India’s alarming ‘Fixed Dose Combination’ problem
“Of all the forms of inequality, injustice in health is the most shocking and inhumane.” - Martin Luther.
Relevance: GS II and III (Governance and Science and Technology)
- Prelims: Antimicrobial resistance (AMR); Drugs & Cosmetics Act, 1940; National Action Plan on Antimicrobial Resistance (NAP-AMR);
- Mains: Governance issues and challenges in the Medical field; Drug Regulatory Framework in India;
Why in the News?
Many unapproved or banned FDCs (Fixed Dose Combination) contain antibiotics is cause for concern given the growing Antibacterial Microbial Resistance in India.
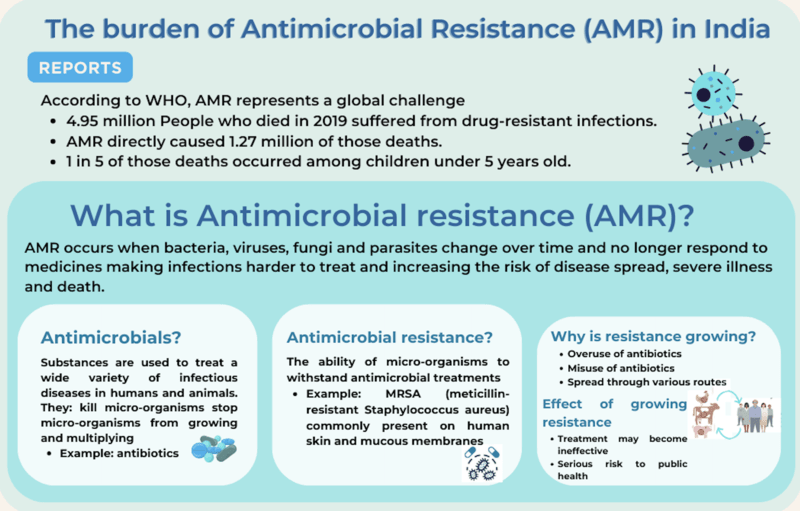
What is a ‘Fixed Dose Combination’ (FDC)?
- FDC drugs are those that contain a combination of two or more Active Pharmaceutical Ingredients (APIs) in a fixed ratio.
- Its Significance: For diseases such as AIDS, it is well documented that FDCs have proven to be very useful in improving patient compliance.
- For example, if a patient has to take three different medications for a particular treatment, she may forget to take one. But if all three medications are combined into one tablet or one syrup, the chance of her forgetting to take one or two of the drugs is reduced.
- The issue with FDCs: However, the drugs may interact to reduce the therapeutic efficacy of each active ingredient, or, worse, the drugs may interact with each other to create a more toxic element, often called metabolites. This is why all FDCs must go through a scientifically designed approval process where such interactions can be evaluated.
- According to Section 26A of the Drugs and Cosmetics Act (1940), the Central Government has powers to regulate, restrict, or prohibit the manufacture, etc., of drugs and cosmetics in the public interest.
Challenges in India:
- Non-effective Regulation: According to the sales data of the Pharmaceutical Industry, even after the 2020 government guideline on FDC, 60.5% of FDCs of antibiotics (comprising 239 formulations) were unapproved and another 9.9% (comprising 39 formulations) were being sold despite being banned in the country.
- Industrial reluctance for FDCs: Pharmaceutical companies in India use these FDCs to escape liability under multiple laws without much concern for public health.
-
- Growing of Irrational medicines: Driven by industrial profit-making interests, and not public health, the Indian pharmaceutical industry introduced anti-inflammatory drugs by combining it with vitamins, anti-histamines with anti-diarrhoeal agents, Penicillin was combined with sulphonamides, and vitamins were combined with analgesics. These were combinations not found in any other country.
- In simple words, due to these wider gaps in regulation, the pharmaceutical industry gets to provide its standards for the government to test its drugs.
- Growing of Irrational medicines: Driven by industrial profit-making interests, and not public health, the Indian pharmaceutical industry introduced anti-inflammatory drugs by combining it with vitamins, anti-histamines with anti-diarrhoeal agents, Penicillin was combined with sulphonamides, and vitamins were combined with analgesics. These were combinations not found in any other country.
- Institutional Loopholes: The Law associated with the Drugs (Prices Control) Order (DPCO), under which the government fixes the prices of individual drugs, does not cover FDCs under the DPCO.
-
- When there are no standards recognized by the law, there is no question of manufacturing “not of standard quality” drugs, and hence there is no possibility of prosecution under the Drugs & Cosmetics Act, of 1940.
- The trap of higher prices: The market and the regulatory structure reward FDCs manufacturers for pseudo-innovation rather than for discovering and developing true innovative medicines. This allows them to charge higher prices for the wrong dose (which is having side effects) until others introduce similar products.
- Corruption: None of the above challenges are possible without doctors who are willing to prescribe such FDCs. While it is tempting to paint all such doctors as corrupt, the fact of the matter is that most doctors wrongly presume that the drug regulator is doing its job when a product is sold on the market.

Way Forward:
- Immediate Action is needed by the Central government:
-
- More than 42 years after the problem was first flagged (in 1978) is an astonishing indictment of the incompetence of the Drug Regulatory Framework in India.
-
-
- The manufacturers selling these FDCs that have not been approved by the DCGI can technically be prosecuted by the Central government for violating the law.
-
- Need for Criminal Prosecutions:
- Instead of ordering criminal prosecutions, the Ministry of Health is constantly invoking its powers under Section 26A to prohibit the manufacture of specific FDCs. This needs to be changed and strictly monitored.
Conclusion: The purpose of the National Policy on Antimicrobial Resistance is to generate accurate and timely estimates of the magnitude and trends in antimicrobial resistance (AMR) burden across the country. It needs to inform treatment guidelines and agendas for decision-making and research, detect emerging problems, and monitor trends to inform global strategies, as well as facilitate the assessment of interventions over time.
BEYOND EDITORIAL:
Measures Taken to Rising Anti-Microbial Resistance in India:
The Union Government of India, aware of the challenges posed by anti-microbial resistance (AMR) in the country, has taken the following measures to address the issue:
- The national programme on AMR containment was launched during the 12th FYP in 2012-17. Under this program, the AMR Surveillance Network has been strengthened by establishing labs in State Medical College. 30 sites in 24 states have been included in this network till 30th March 2021.
- The National Action Plan on Antimicrobial Resistance (NAP-AMR) focusing on the One Health approach was launched on 19th April 2017 to involve various stakeholder ministries/departments. Delhi Declaration on AMR– an inter-ministerial consensus was signed by the ministers of the concerned ministries pledging their support in AMR containment. In line with NAP-AMR three states have launched their state action plan:
- Kerala has launched KARSAP
- Madhya Pradesh has launched MP-SAPCAR
- Delhi has launched SAPCARD
- AMR Surveillance Network: ICMR established AMR Surveillance and Research Network (AMRSN) in 2013, to generate evidence and capture trends and patterns of drug-resistant infections in the country. This network comprises 30 tertiary care hospitals, both private and government.
- AMR Research & International Collaboration: ICMR has taken initiatives to develop new drugs /medicines through international collaborations to strengthen medical research in AMR.
- ICMR along with the Research Council of Norway (RCN) initiated a joint call for research in antimicrobial resistance in 2017.
- ICMR along with the Federal Ministry of Education and Research (BMBF), Germany has a joint Indo-German collaboration for research on AMR.
- Initiatives to control overuse or misuse of antibiotics:
- ICMR has initiated an antibiotic stewardship program (AMSP) on a pilot project basis in 20 tertiary care hospitals across India to control the misuse and overuse of antibiotics in hospital wards and ICUs.
- On the recommendations of ICMR, DCGI has banned 40 fixed-dose combinations (FDCs) that were found inappropriate.
- ICMR worked in collaboration with the Indian Council of Agriculture Research, the Department of Animal Husbandry, Dairy and Fisheries, and the DCGI to ban the use of Colistin as a growth promoter in animal feed in poultry.
Previously Issued Guidelines:
- Year 2019: ICMR issued the Treatment Guidelines for Antimicrobial Use in Common Syndromes”.
- ICMR has developed evidence-based treatment guidelines for the treatment of ten syndromes of infections. It aims to rationalize the usage of antibiotics on Essential Medicines Formulary (EMF) and to establish consistency in the treatment of various infectious conditions.
- The year 2020: National Guidelines for Infection Prevention and Control in Healthcare Facilities was released by MoHFW in Jan 2020.
- The year 2023: The Union Health Ministry has banned 14 Fixed Dose Combination drugs including Nimesulide and Paracetamol dispersible tablets and Chlorpheniramine Maleate and Codeine syrup citing there is "no therapeutic justification" for these medicines and that they may involve "risk" to people.
Mains PYQs
Q. What do you understand by Fixed Dose drug Combinations (FDCs)? Discuss their merits and demerits. (2013)
Q. Can overuse and free availability of antibiotics without Doctor’s prescription, be contributors to the emergence of drug-resistant diseases in India? What are the available mechanisms for monitoring and control? Critically discuss the various issues involved. (2014)
Q. How is the Government of India protecting traditional knowledge of medicine from patenting by pharmaceutical companies? (2019)
Prelims PYQs
Q. Which of the following are the reasons for the occurrence of multi-drug resistance in microbial pathogens in India?
- Genetic predisposition of some people
- Taking incorrect doses of antibiotics to cure diseases
- Using antibiotics in livestock farming
- Multiple chronic diseases in some people
Select the correct answer using the codes given below:
(a). 1 and 2 only
(b). 2 and 3 only
(c). 1, 3 and 4
(d). 2, 3 and 4

